

We'll take an introductory look at the new and controversial process of umbilical cord blood banking.
Umbilical cord blood banking is a process that many newly expecting parents have never heard of, but more than likely, it will be addressed towards the end of their pregnancy. The issue of umbilical cord blood banking will continue to become more widely discussed in the next several years as stem cell research becomes a more hotly debated and funded project.
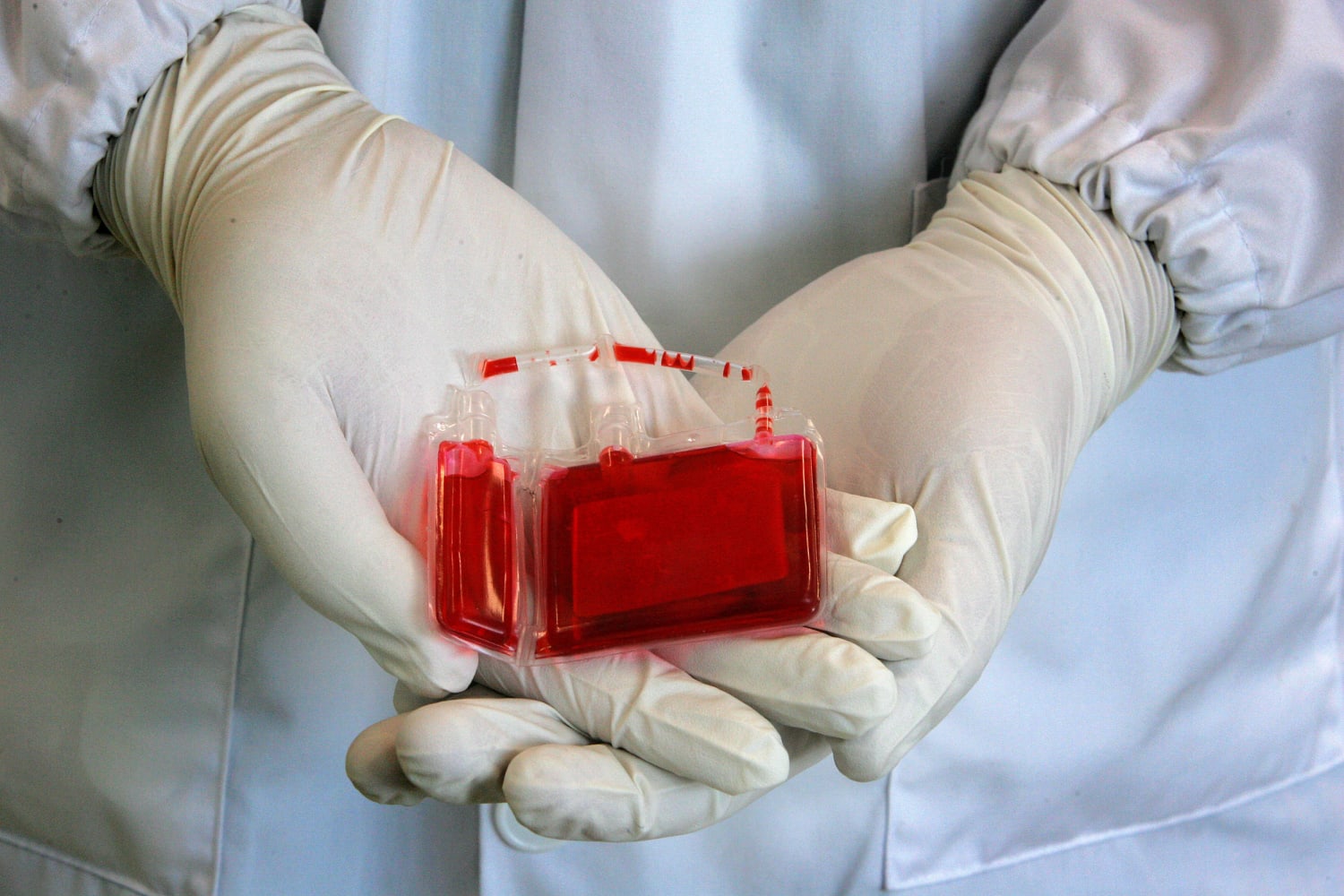
What is umbilical cord blood banking, exactly? First of all, we'll explain the process. There are two different processes to collect the cord blood. In one process, after the fetus has been delivered and before the placenta has been delivered, the attending physician draws the cord blood through a syringe while the umbilical cord is clamped. In the other process, the cord blood is extracted from the umbilical cord after the placenta has been delivered. Both methods yield between 60 to 120 mL of umbilical cord blood.
What happens after the umbilical cord blood is extracted? After the umbilical cord blood is collected, it is shipped to a lab at a cord blood bank for testing and processing. A battery of tests is conducted on the blood, stem cells are extracted, and the sample is prepared for cryogenic freezing at -385 Fahrenheit.
What is the value of umbilical cord blood banking? Umbilical cord stem cells have been used for genetic disorders and diseases such as leukemia and lymphoma, as well as several types of anemia. Unlike bone marrow transplants, studies suggest that transplanted stem cells need not perfectly match the recipient. In essence, the stem cells extracted through umbilical cord blood banking are a type of biological insurance not only for the baby but for the baby's family, as well as the general public.
So why is this process controversial? A common reason that stems cell research is cited as controversial is that the human race is "playing God", among other ethical arguments. Also, the use, collection, and storage of umbilical cord blood and stem cells is still experimental, in its stage of infancy. Currently, the Food and Drug Administration (FDA) does not regulate umbilical cord blood banking, although they do regulate the use of most human tissue.
Another sticking point for many families is the cost of the process. While the idea of having a strong weapon against future diseases is priceless, the process does come with a price. Family banking fees currently average about $1500, along with an annual storage fee of $100-$150. Health insurance companies likely won't cover the cost of umbilical cord blood banking until the process is proven as a safe and cost-effective way to combat long-term illness. Weighing the costs of the process versus the odds that someone in your family would ever need the cells is pertinent, if your family has a history of disease, it may be worth the cost. If your family has a clean health history, is it worth the cost?
Just like the more common way of blood donation, public umbilical cord blood banking is now available for free. However, just like the common type of blood donation, there is no guarantee that "your" blood will be available in case you need it. Public umbilical cord blood banking helps to populate the supply and variety in a public blood bank, but when you donate your family's blood, you sign off all rights to it. This is another ethical issue, as a family no longer has a right to what was theirs.
Parents owe it to themselves to research the process of umbilical cord blood banking, whether they choose to use a private umbilical cord blood bank or a public one, or whether they choose to decline participation in the process. This issue will likely command more public attention as the cord blood banking process is refined and proven, and as stem cell research becomes a hot topic in the coming years.
Note: Please also check your spam or junk email folder.